Greetings! I would certainly call last month the best for “getting things done” so far in this project.
Tuesday, July 16th, was the first lab day of our attempt to isolate cellulose nanofibers (CNFs) from our dried and partially grinded Sargassum muticum samples. I worked with Dr. Joel Smith, a chemistry professor who graciously lent us his lab space and expertise, and Tracey Bell, a graduate biology student. The first step involved was bleaching the algae.

An issue we more deeply realized through the day was the lack of rigor in the Cengiz article. There were minor errors (such as 2M and 4 mol/L switched around, an anhydrous solution in water, testing pH of a non-liquid substance, and a lack of volumes given for some refluxing solutions), but we came across larger ones as we proceeded with the bleaching. It’s likely that the paper’s reviewers were not largely familiar with chemistry, which is how these errors slipped through. There are other articles covering CNF extraction that I have included in my literature review, including ones published in more specialized chemistry journals, although they involve a more complex process called TEMPO-mediated oxidation. I wanted to stick to the seemingly more simple Cengiz method for this experiment.
I decided to try and process 100g of algae, instead of the 20g used in the Cengiz article, so that there would be ample CNFs available if I proceeded into concrete testing or other uses this fall. We multiplied the needed water and NaOCl (the active ingredient in bleach) amounts by 5. After adding the water and bleach to a 2L round-bottom flask submerged in hot oil, we added the dried and grinded algae (including dust and some larger particles). It began bleaching quickly as the temperature in the submerged bulb approached 100*C.

However, the heterogeneous reaction began producing large amounts of foam and gas that eventually spilled out of the long refluxer (essentially, a device that allows steam to condense back into the solution) attached to the top of the bulb, creating a mess. This was a possible reaction that should have been addressed in the paper. Additionally, a large number of the fibers did not even touch the near-100*C solution, because of the foam lifting them away from the solution (as seen in the images).

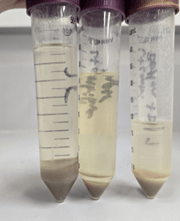
We then distributed the solution and fibers into three different flasks (one with mostly bleached algae, the other two with partially bleached algae). Very little further progress was made on further bleaching the algae from this point, even after leaving the algae in the solutions overnight. The flask in the farthest left image has the most successfully bleached algae, and the other flasks have the less successfully bleached algae.

Since we had spent around 3 hours in the lab by this point, we decided to take the most well-bleached algae solution, strain out the liquid, and try to dry and further pulverize the algae, which may improve the bleaching process and prevent hiccups in the further steps of the experiment.

We returned to the lab a week later with about 12g of successfully bleached and pulverized algae. We refluxed it in 120 mL of 2M sodium hydroxide (NaOH) solution for 2 hours, with a round-bottom flask in an oil bath set to 130*C, to remove the proteins from the algae structure. Thankfully, the algae did not erupt in foam as severely this time around.

We then had serious problems with filtering the algae out of the solution for the next step. When using larger pieces of algae (for the bleaching process we attempted last week), the draining process was rather easy, but it became almost impossible when we used the more fine algae, even with an extremely powerful vacuum-filter apparatus.
Additionally, the study calls for rinsing the algae in water to neutralize it after the NaOH bath, but we realized it was very tedious to lower the pH with this method. We wound up returning the small amount of algae we extracted back in the solution and added enough 3M HCl (very low pH) to neutralize it. We noted that the color of the solution changed with the pH, with it clarifying as we neutralized it. Of course, we ran back into the issue of removing the algae from the solution when we were finished.

Ms. Bell suggested that we centrifuge the algae samples over at her biology lab, since centrifuges work well at separating biological items from solution. Essentially, the centrifuges spin the tubes of algae at an angle, causing the heavier algae to sink to the bottom. We centrifuged for about 10 minutes total at 8000 RPM and had successful extraction of the algae from the solution.

We then refluxed the algae in a 120mL, 4:2:1 chloroform-methanol-distilled water v/v solution for 20 minutes, using a round-bottom flask submerged in an oil bath set to 150*C. This step serves to remove the oil and pigments from the algae structures. Separation of the different reagents was clearly noticeable when not being stirred. We successfully centrifuged the algae out of the solution after the refluxing completed.
Finally, we refluxed the remaining algae in 120 mL of 2M HCl for about 18 hours (4PM to 10AM the following day), using a round-bottom flask in an oil bath set to 110*C. Ideally, this final step would break down the remaining cellulose into its nanofiber components.

We then centrifuged the solution and collected the chunks of (presumably) cellulose fibers/nanofibers. We are letting them dry over the next 3 weeks in a 40*C heating device (1 week of drying is recommended in the article).
I look forward to moving onto the next step of characterizing the algae (through a scanning electron microscope, x-ray diffraction, and FT-IR), since these will provide the visible results of whether our extraction process worked properly. If successful, we will have accomplished the first documented extraction of cellulose nanofibers from Sargassum muticum, a discovery that can have far-reaching uses! And, of course, it will be very satisfying to present these results at the President’s Showcase.